(Adnkronos) - Commercial Collaboration Follows News of Nerivio®'s Expanded CE Mark Approval as Novel Non-Drug, Dual-Use Migraine Treatment for Adolescents and Adults
NETANYA, Israel, Nov. 14, 2023 /PRNewswire/ -- Theranica, a neuromodulation therapeutics company, and Dr.Reddy's Laboratories Ltd.("Dr.
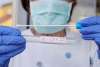
This comes after Theranica announced in August this year that Nerivio®, a novel, drug-free REN (Remote Electrical Neuromodulation) wearable for migraine, has received an expanded CE mark approval under MDR European regulation as a dual-use (acute and/or prevention) migraine treatment for adults and adolescents.
The commercial agreement between Theranica and various European subsidiaries of Dr. Reddy's includes licensing fees and additional payments in return for the exclusive rights to market Nerivio® in various European countries. Dr. Reddy's will manage marketing, sales, and local support aspects of the Nerivio® commercialization across Europe, starting with Germany and gradually expanding throughout the European countries mentioned above. This follows an earlier agreement between Theranica and Dr. Reddy's regarding the commercial collaboration in India, for Nerivio®.
"This agreement carries long-awaited news for millions of people with migraine all over Europe," says Ronen Jashek, Theranica COO. "After the welcoming acceptance of Nerivio in the USA and following numerous requests from neurologists and patients in Europe, this commercial collaboration with Dr. Reddy's makes Nerivio available to even more patients around the world. Migraine is a leading cause of disability worldwide, affecting 100 million adolescents and young adults. Early intervention with both preventive and acute treatment is imperative to restrain the disease from escalating in adulthood. We put special emphasis on bringing this innovative therapy to underserved populations, such as adolescents."
"Dr. Reddy's commercial collaboration with Theranica perfectly aligns with our commitment to address unmet patient needs," says Patrick Aghanian, CEO of Generics Europe, Dr. Reddy's Laboratories. "We are excited to bring a drug-free migraine treatment option to European patients, which could make a difference to migraine patients' quality of life."
About Nerivio
Controlled by a smartphone app and self-administered, the Nerivio REN wearable is a complete migraine care treatment that wraps around the upper arm and uses sub-painful Remote Electrical Neuromodulation (REN) to activate nociceptive nerve fibers in the arm. These fibers send signals that trigger a descending pain management mechanism in the brain called conditioned pain modulation (CPM), which turns off migraine pain and associated symptoms without medication. In simpler terms, the upper arm is stimulated to unleash a natural process in the brain to abort or relieve migraine headaches and other associated symptoms. Each treatment lasts 45 minutes and is applied every other day for prevention or at the start of a migraine attack for acute treatment.
About Theranica
Theranica is a prescribed digital therapeutics company dedicated to creating effective, safe, affordable, low-side-effect therapies for idiopathic pain conditions. The company's award-winning flagship wearable, Nerivio®, is the first FDA-cleared prescribed migraine REN Wearable for acute and/or preventive treatment of migraine Nerivio has been used in more than 650,000 migraine treatments in the US, including by adolescents and veterans living with migraine. Theranica is expanding its proprietary technology to develop solutions for additional idiopathic pain conditions. Learn more by visiting our websites, theranica.com and nerivio.com, and following us on LinkedIn, Twitter, Instagram and Facebook.
#NoMatterWhat
Theranica ContactRonen Questo indirizzo email è protetto dagli spambots. È necessario abilitare JavaScript per vederlo.+972-72-390-9750
Media ContactV.A. LopesGrey Matter Questo indirizzo email è protetto dagli spambots. È necessario abilitare JavaScript per vederlo.
Photo - https://mma.prnewswire.com/media/2274150/Nerivio_adolescent_use_min.jpgLogo - https://mma.prnewswire.com/media/2011113/Theranica_Logo.jpg
View original content:https://www.prnewswire.co.uk/news-releases/theranica-enters-into-agreement-with-dr-reddys-for-commercializing-nerivio-in-europe-301986229.html